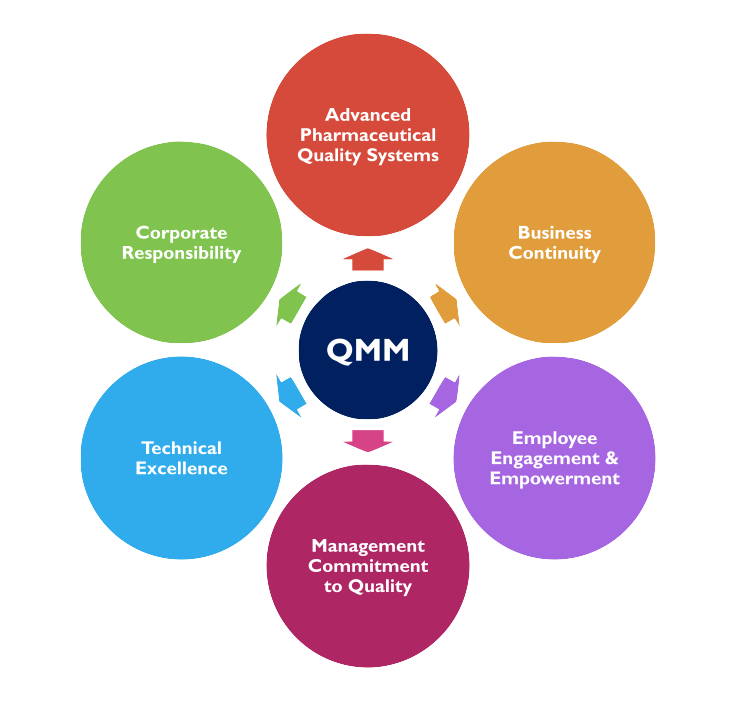
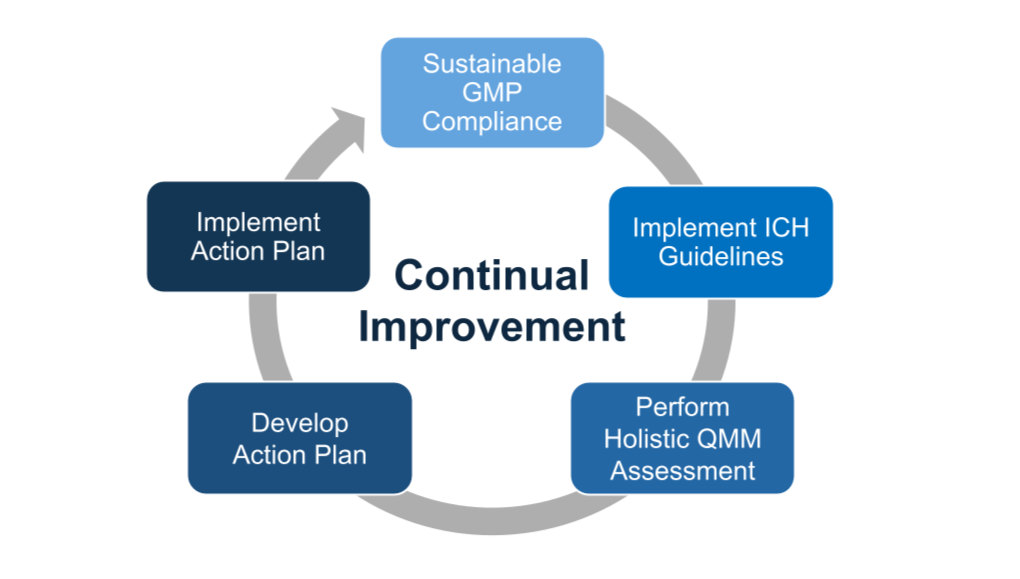
Our Experience Providing QMM Assessment Services
Case Study
FDA engaged Shabas through a competitive selection process as a prime contractor to
lead the QMM Pilot for API Manufacturers (non-US). Eight API manufacturers from
Asia, Europe and South America volunteered for this initiative. Under FDA’s guidance,
Shabas conceptualized, developed and administered a QMM Assessment protocol
specifically designed for facilitated assessment of the API Manufacturers.
Problems and Solutions
- Being unique, the QMM Assessment protocol for the API Manufacturers had to be designed from the ground-up to meet FDA’s requirement to gauge a manufacturing site’s ability to produce quality products consistently and reliably.
- The Shabas team developed an innovative assessment framework and a detailed analytical tool by leveraging insights from pharma and other industries that encompassed FDA-suggested practice areas and topics.
- The tool was administered virtually to the participating sites (with FDA as a silent observer) via facilitated assessment and results communicated to them (including cross-site benchmarking) using automated data analysis and creative reporting mechanisms.
Impact
- Shabas facilitated assessments using this comprehensive QMM Assessment tool was well received by the FDA global QMM pilot participants and was a factor in their positive views of the QMM program.
- Due to the success of and positive feedback for the Shabas-designed tool during the FDA pilot program, Shabas is now uniquely well-positioned to offer QMM assessment services to pharmaceutical companies globally.
Feedback from Pilot Participants was Overwhelmingly Positive
- Sites received identified areas of strengths and weaknesses.
- Topic areas previously unconsidered or unaddressed by sites were highlighted as part of the assessment.
- The assessment demonstrated an overview of site operations and how those operations can be improved moving forward, providing sites with an opportunity to reflect and continually improve their processes.
- Overall, the sites appreciated the holistic big-picture approach of the QMM assessment, which allowed their systems to be examined beyond traditional audit requirements.
References
1 Office of Pharmaceutical Quality (OPQ), CDER, FDA “White Paper: Quality
Management Maturity: Essential for Stable U.S. Supply Chains of Quality
Pharmaceuticals”, (2022) https://www.fda.gov/media/157432/download
2 FDA, Drug Shortages: Root Causes and Potential Solutions A Report by the Drug Shortages Task Force 2019, Report, fda.gov, updated Feb 21, 2020
2 FDA, Drug Shortages: Root Causes and Potential Solutions A Report by the Drug Shortages Task Force 2019, Report, fda.gov, updated Feb 21, 2020