Shabas Is A Specialist Provider of Quality Management Maturity (QMM) Services to the Pharmaceutical Industry
A scientific and management consulting provider with expertise in drug development R&D, drug manufacturing, stockpiling and distribution, quality assurance, and regulatory compliance. Our approach is informed by our experience leading the FDA QMM Pilot for Foreign API Manufacturers, supporting US federal government life-sciences organizations, and complemented by the collective experience of SMEs who have extensive past affiliation with Big 4 Global Management Consulting firms, National Regulatory Authorities, Biologics and Small Molecules Drug Substance/Drug Product companies and Medical Device manufacturers.
What is QMM?
- Quality Management Maturity (QMM) goes beyond simple regulatory compliance and is a rating system that shows that a pharma manufacturer’s site business processes are robust, integrated with quality objectives, being continually improved, and most importantly, are reliable. Going beyond GMP and ICH Q10, QMM is the next chapter in pharmaceutical quality standards.
- Shabas has developed and launched a unique QMM Assessment and Consulting program for the Pharma Industry and specifically for Drug Substance/API Producers after a successful FDA QMM Pilot for Foreign API Manufacturers.
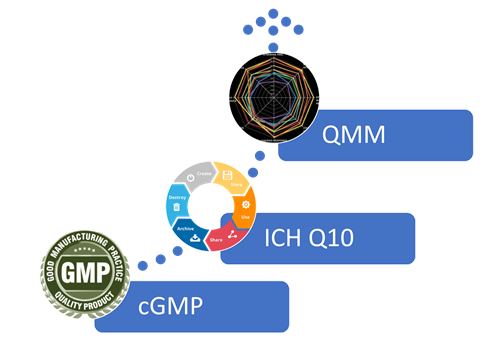
- According to the FDA, Quality management maturity (QMM) is the state attained when drug manufacturers have consistent, reliable, and robust business processes to achieve quality objectives and promote continual improvement.
- An FDA-assigned QMM rating will allow pharma purchasers to consider a manufacturing site’s state of quality into their purchasing decision in addition to pricing.
- An adverse QMM rating can therefore be not only detrimental to the reputation and brand of the manufacturer but also affect its bottom line.
Shabas has extensively researched the pharmaceutical and other quality advancing industries to identify key foundational competencies that form the bedrock of QMM for an organization aspiring to be “quality management mature”. Our four pillars are:
- Compliance management: The ability to consistently meet and/or exceed compliance with applicable laws, regulations, and industry best practices.
- Risk management: The ability to identify and manage organizational risks and build resilience
- Quality culture: The development and maintenance of organizational mindset and behaviors that value continuous learning, improvement, and customer satisfaction
- Sustainability: The ability to successfully secure and manage appropriate technology, infrastructure, human capital, suppliers, customers, institutional knowledge, and other resources to sustain operations
What are the benefits of QMM?
Pharma manufacturers of any size can benefit from a QMM assessment, some of these benefits are…
Improved investment decisions
Risk-based redundancies
- Enhanced operations for resiliency, workforce efficiency, collaboration, etc.
A robust Quality Culture
Shabas QMM consulting solution provides greater visibility to a site’s business processes, improves synergy between functional areas, and provides a pathway to progressively fit Quality into a manufacturing site’s strategic plan.
Shabas can help your company assess, re-imagine, and transform your manufacturing site operations based on QMM principles and practices to boost brand, organizational reputation, and value.
How can Shabas help?
Shabas QMM consulting solution provides greater visibility to a site’s business processes, improves synergy between functional areas, and provides a pathway to progressively fit Quality into a manufacturing site’s strategic plan.
Shabas can help your company assess, re-imagine, and transform your manufacturing site operations based on QMM principles and practices to boost brand, organizational reputation, and value.
Go beyond compliance, contact us today.
SUPPORT
Contact
- 11166 Fairfax Blvd., Suite 310, Fairfax, VA 22030
- 703-763-7685
- 703-763-7685
- contact@shabas.net