Pharmaceutical Industry Consulting
Shabas is The Premier Provider of Quality Management Maturity (QMM) Consulting Services for the Pharmaceutical Industry
Shabas is a scientific and management consulting provider with deep expertise in drug development R&D, drug manufacturing quality, and medical logistics. The Shabas approach to QMM is informed by experience leading the FDA QMM Pilot for Foreign API Manufacturers, supporting US federal government life-sciences organizations, and conducting a multi-site QMM readiness assessment in 2025 with a major multinational drug manufacturer. Shabas is the leading partner for pharmaceutical manufacturers seeking to begin or sustain internal quality management maturity initiatives. With comprehensive collective knowledge, including SMEs with decades of experience with Big 4 Global Management Consulting firms, National Regulatory Authorities, Biologic and Small Molecule Drug Substance/Drug Product and Medical Device manufacturers, Shabas will deliver actionable insights and strategies for your organization to go beyond compliance.
Shabas Solutions is proud to be a pioneer in the FDA’s Quality Management Maturity (QMM) initiative—helping advance a more resilient, transparent, and reliable pharmaceutical and medical product supply chain.
As a federally recognized U.S. consulting firm supporting public-sector quality and regulatory initiatives, Shabas supports organizations in building, assessing, and operationalizing quality management systems that align with FDA QMM principles, global quality standards, and regulatory expectations. Our work focuses on moving beyond compliance toward true maturity—where quality is embedded, measurable, and sustainable.
This video highlights our role in supporting the evolution of QMM, our commitment to data-driven quality excellence, and our mission to partner with government and industry to strengthen public health outcomes.
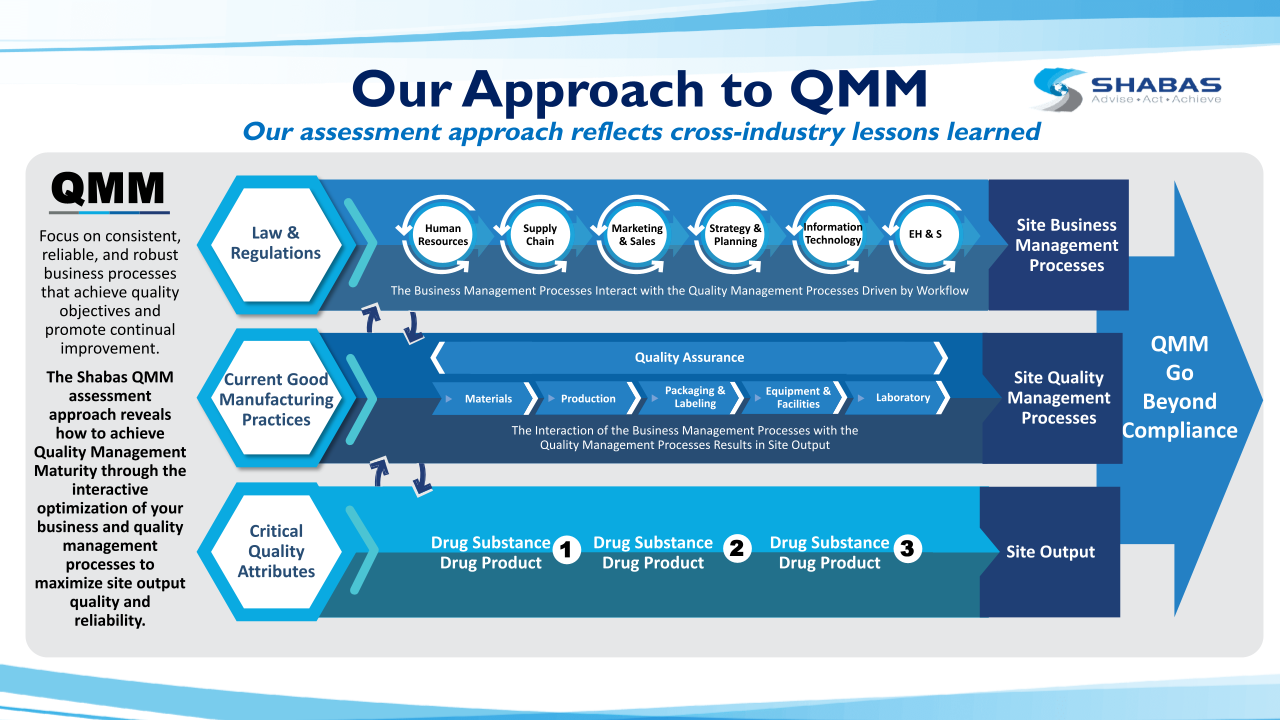
What is QMM?
- Quality Management Maturity (QMM) is the state attained when drug manufacturers have consistent, reliable and robust business processes to achieve quality objectives and promote continual improvement per the FDA. Office of Pharmaceutical Quality (OPQ), CDER, FDA “White Paper: Quality Management Maturity: Essential for Stable U.S. Supply Chains of Quality Pharmaceuticals”, (2022) https://www.fda.gov/media/157432/download
- QMM goes beyond cGMP regulatory compliance and ICH Q10 and is the next evolution of pharmaceutical quality standards.
- QMM was identified by the FDA as a potential solution to resolve ongoing drug shortages in which quality issues were identified as the leading cause for the majority of shortages that occurred. https://www.fda.gov/drugs/drug-shortages/report-drug-shortages-root-causes-and-potential-solutions
- In 2020, the FDA initiated two QMM pilot programs for domestic drug product manufacturers and foreign drug substance manufacturers in collaboration with contractors to inform them on QMM within industry.
- Shabas successfully led the Global drug substances manufacturer QMM pilot program developing an assessment protocol in collaboration with the FDA. This protocol has been further refined in accordance with FDA’s published guidelines and is now used as the basis for Shabas’ QMM Assessment and Consulting program for the Pharma Industry, specifically tailored for both drug substance and drug product manufacturers.
The Shabas QMM Assessment Protocol
Shabas has extensively researched the pharmaceutical and other quality-advancing industries to identify the key tenets that inform the Shabas Quality Management Maturity Assessment approach. These principles are well aligned with the published guidance that the FDA considers to be central to the foundation of QMM for an organization aspiring to be a “quality mature organization.”
2 FDA Presentation, Harouaka, D., Quality Management Maturity (QMM), April 12, 2023. https://www.fda.gov/media/168940/download
3 The ellipses signify that additional topics may be addressed in the finalized FDA QMM Guidance, therefore this graphic provides only an initial snapshot of expected topics, not a complete list.
What are the benefits of QMM?
Pharma manufacturers of any size can benefit from a QMM assessment.
Readiness for a future FDA QMM Program
Readiness for a future FDA QMM Program: Achieve readiness for a future FDA QMM Program by preparing for high ratings now. High initial QMM assessment ratings in the FDA program could increase competitive advantages in the marketplace and potentially qualify for regulatory flexibilities.
Operational Efficiency
Optimized and robust business processes leading to streamlined operations and the production of consistent and reliable high-quality products, resulting in increased productivity, reduced cost of quality and a higher return on investment.
Continual Improvement
Identify and benchmark strengths and areas for continual improvement, providing insights into functions not typically captured as part of routine audits or regulatory inspections.
Customer Satisfaction
Build a flexible and adaptive supply chain that contributes to resiliency and greater customer satisfaction in meeting market demands.
Strategic Risk Management
Empower improved risk-based decision-making to ensure the appropriate investment and allocation of resources.
Prepare for the Future of QMM
- The FDA has recently launched a second round of their voluntary Quality Management Maturity Prototype Assessment Protocol Evaluation Program with drug manufacturers in 2025 to gain additional experience and further refine their pilot assessment protocol and process
- This program, which is anticipated to go live in the near future , is intended to assign ratings to manufacturers based on their Quality Management Maturity, which can be used by pharma purchasers as part of their consideration when making purchasing and pricing decisions.
- Possessing robust QMM ratings can assist in highlighting a drug manufacturer as especially dedicated to continual improvement and process optimization resulting in the reliable supply of high-quality products, positively differentiating it from its competitors.
- Now is the time to act. Early adopters of the QMM assessment process can address adverse ratings in advance and avoid negative impacts, such as diminished reputation and weakened financial prospects, before participating in the FDA QMM program following its initiation.
QMM Assessment Services
Full Cycle and Comprehensive Assessment Services
Read More
QMM Development Services
Strategy and Operations Services – go beyond GMP
Read More